The impact of fasting toward oxidative stress marker in the liver and plasma of new zealand white rabbit
Abstract
Background: Fasting may increase the activity of endogenous antioxidants and protect against oxidative stress. However, the effects of different fasting durations on the liver have not been reported.
Objective: The purpose of this study is to determine the effect of intermittent and prolonged fasting on oxidative stress markers in the liver tissue and plasma of New Zealand White rabbits.
Methods: New Zealand White rabbits were divided into three groups: control, intermittent fasting (IF), and prolonged fasting (PF), with each group consisting of five rabbits. The control group was provided with food ad libitum; the IF group fasted for 16 hours, while the PF group fasted for 40 hours, followed by an eight-hour non-fasting period for six days. In liver tissue and plasma, oxidative stress indicators (catalase, carbonyl, GSH) were evaluated.
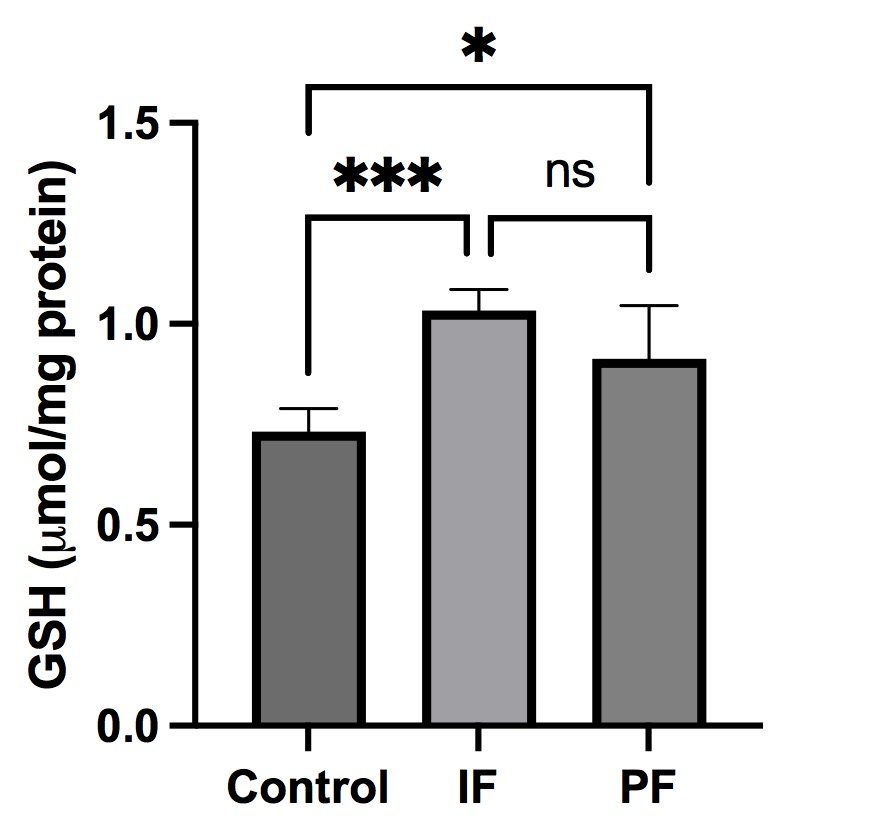
Results: In the IF group, liver GSH was significantly higher than in the control group. However, neither liver carbonyl nor catalase levels changed significantly in the IF group. In the IF group, plasma carbonyl was significantly lower than in the PF group. In addition, there was no significant differences between groups in plasma catalase and GSH levels.
Conclusion: Intermittent fasting and prolonged fasting could significantly increase liver GSH levels of New Zealand White rabbits. In addition, intermittent fasting is more effective than prolonged fasting at preventing oxidative stress.
References
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:8416763. https://doi.org/10.1155/2017/8416763
Ligouri I, Russo G, Curcio F, Bulli G, Aran L, Della-morte D, et al. Oxidative stress , aging , and diseases. Clin Interv Aging. 2018;13:757-72. https://doi.org/10.2147/CIA.S158513
Gonos ES, Kapetanou M, Sereikaite J, Bartosz G, Naparto K, Grzesik M, et al. Origin and pathophysiology of protein carbonylation, nitration, and chlorination in age-related brain diseases and aging. Aging. 2018;10(5):868-88. https://doi.org/10.18632/aging.101450
Luceri C, Bigagli E, Femia AP, Caderni G, Giovannelli L, Lodovici M. Aging related changes in circulating reactive oxygen species (ROS) and protein carbonyls are indicative of liver oxidative injury. Toxicoloy Reports. 2017;5:141-5. https://doi.org/10.1016/j.toxrep.2017.12.017
Dzibula T, Butterfield DA. Oxidative stress and biomaterials. London: Academic Press Elsevier.2016. 389 p.
Li S, Tan H, Wang N, Zhang Z, Lao L, Wong C, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16:26087-124. https://doi.org/10.3390/ijms161125942
Walsh ME, Shi Y, Van Remmen H. The effects of dietary restriction on oxidative stress in rodents. Free Radic Biol Med. 2014;66:88-99. https://doi.org/10.1016/j.freeradbiomed.2013.05.037
Glorieux C, Calderon PB. Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol Chem. 2017;398(10):1095-108. https://doi.org/10.1515/hsz-2017-0131
Flohe L. Glutathione. Boca Raton: Taylor & Francis; 2019. 385 p.
Vaiserman AM, Lushchak OV, Koliada AK. Anti-aging pharmacology: promises and pitfalls. Ageing Research Reviews. 2016;31:10- 27. https://doi.org/10.1016/j.arr.2016.08.004
Hanjani NA, Vafa M. Protein restriction, epigenetic diet, intermittent fasting as new approaches for preventing age-associated diseases. Int J Prev Med. 2018;9:58. https://doi.org/10.4103/ijpvm.IJPVM_397_16
Hipkiss AR, Baye E, Courten B.Carnosine and the processes of ageing. Maturitas. 2016;93:28-33. https://doi.org/10.1016/j.maturitas.2016.06.002
Golbidi S, Daiber A, Korac B, Li H, Essop MF, Laher I. Health benefits of fasting and caloric restriction. Curr Diab Rep. 2017;17(12):123. https://doi.org/10.1007/s11892-017-0951-7
Chausse B, Vieira-lara MA, Sanchez AB, Medeiros MHG, Kowaltowski AJ. Intermittent fasting results in tissue-specific changes in bioenergetics and redox state. PLoS One. 2015;10(3) :1-13. https://doi.org/10.1371/journal.pone.0120413
Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic Biol Med. 2003;35(6):626-35. https://doi.org/10.1016/S0891-5849(03)00388-5
Smyers ME. Dietary restriction, physical activity, and metabolism: potential role of intermittent fasting for reducing obesity [dissertation]. Parkway : Proquest LLC; 2019.
Marinho TS, Ornellas F, Barbosa-da-Silva S, Mandarim-de-Lacerda CA, Aguila MB. Beneficial effects of intermittent fasting on steatosis and inflammation of the liver in the mice fed a high-fat or a high-fructose diet. Nutrition. 2019;65:103-12. https://doi.org/10.1016/j.nut.2019.02.020
Bollineni RC, Fedorova M, Blüher M, Hoffmann R. Carbonylated plasma proteins as potential biomarkers of obesity induced type 2 diabetes mellitus. J Proteome Res. 2014;14:5081-93. https://doi.org/10.1021/pr500324y
Hardiany NS, Karman AP, Calista ASP, Anindyanari BG, Rahardjo DE, Novira PR, et al. The effect of fasting on oxidative stress marker in the vital organs of new Zealand white rabbit. Rep. Biochem. Mol. Biol. 2022;11(2):190-9.
Hardiany NS, Mulyawan W, Wanandi SI. Correlation between oxidative stress and tumor grade in glioma cells from patient in Jakarta. Med J Indones. 2012;21:122-7. https://doi.org/10.13181/mji.v21i3.492
Hardiany NS, Asa AA, Safirina D, Mulyawan W. The effect of intermittent hypoxia hypobaric exposure on reduced-glutathione level in rat lung and renal tissues. Adv Sci. Lett. 2018;24(8):6249-51. https://doi.org/10.1166/asl.2018.12704
Hardiany NS, Sucitra, Paramita R. Profile of malondialdehyde (MDA) and catalase specific activity in plasma of elderly woman. HSJI. 2019;10(2):132-6. https://doi.org/10.22435/hsji.v12i2.2239
Burgos-Morón E, Abad-Jimënez Z, de Marañon AM, Iannantuoni F, Escribano-López I, López-Domènech S, et al. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. J Clin Med. 2019;8:1385. https://doi.org/10.3390/jcm8091385
Wu P, Wang A, Cheng J, Chen L, Pan Y, Li H, et al. Effects of starvation on antioxidant-related signaling molecules oxidative stress, and autophagy in juvenile chinese perch skeletal muscle. Mar Biotechnol. 2020;22(1):81-93. https://doi.org/10.1007/s10126-019-09933-7
Cherif A, Roelands B, Meeusen R, Chamari K. Effects of intermittent fasting, caloric restriction, and ramadan intermittent fasting on cognitive performance at rest and during exercise in adults. Sports Med. 2016;46:35-47. https://doi.org/10.1007/s40279-015-0408-6
Wilhelmi de Toledo F, Grundler F, Goutzourelas N, Tekos F, Vassi E, Mesnage R, et al. Influence of long-term fasting on blood redox status in humans. Antioxidants (Basel). 2020;9(6):496. https://doi.org/10.3390/antiox9060496
Vázquez-Medina JP, Zenteno-Savín T, Forman HJ, Crocker DE, Ortiz RM. Prolonged fasting increases glutathione biosynthesis in postweaned northern elephant seals. J Exp Biol. 2011;214(Pt 8):1294-9. https://doi.org/10.1242/jeb.054320
Copyright (c) 2022 Authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.







